Intermolecular forces
The force of attraction present between the molecules to bring them closer or to bind them is called intermolecular forces.
Types of intermolecular forces
There are two types of intermolecular forces- Dipole-Dipole forces
- Hydrogen bonding
Dipole-Dipole forces
In polar covalent molecules each atom carries partial positive and partial negative charge is called dipole. The force of attraction present between them having dipole is called dipole-dipole interaction.In dipole-dipole interaction partial positive end attracts partial negative end of the neighboring molecule. Such as
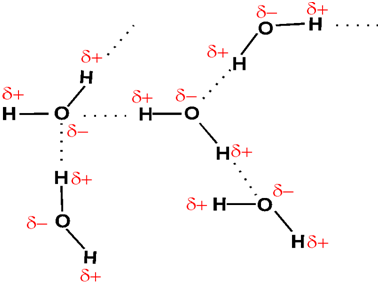
Hydrogen bonding
The force of attraction present between partial positive hydrogen of a polar molecule and partial negative of the same polar molecule is called hydrogen bonding.This bond is represented by dotted line. The molecules having hydrogen bond have high melting and boiling point. Thus H2O has a hydrogen bond therefore its boiling point is 100 centigrade and H2S has no hydrogen bond therefore its boiling point is 60 centigrade.
Due to hydrogen bond ice has lowest density than water therefore ice floats on the surface of water.