|
| Download Registered Softwares free |
Download Registered Softwares free
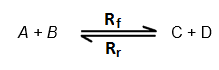
Today, in this article, On the Behalf of www.Onllogy.com, i Khushal Khan Nasar am going to teach you about How to Download The registered Softwares for free. Its not so difficult, its very easy and simple. The ScreenShot is as given below of the registered Softwares, which you can download for free. i-e
Related Posts
- Einstein's Theory of relativity in Urdu, Hindi
- Happy New Year HD Wallpapers 2015
- Start Making Money Online
The Following Steps which are included to download the registered softwares are as follow
- Go to the ninite.com
- After accessing the ninite, you will see the all king of softwares lists including the web browsers softwares messaging softwares media softwares and many more softwares too.
- There will be a small checkbox in the start of every software. So, click on the checkbox of the software you want to download (you can select even the multiple softwares too.)
- After selecting the softwares just click on the Get Installer button located below. i-e
- After clicking on it, the installer will be download on you PC.
- After downloading, Open it and wait for installing the softwares you selected.
- After installing the softwares, you can enjoy it fully as these softwares are registered and do not want any registration from you people.
Thanks for reading the article and now subscribe us for more news updates and feedback us on social media. and contact us for free for any type of question.








.jpg)
.jpg)









.jpg)