|
| Sir William Crooks |
In
since 1895 Sir William Crooks performed an experiments by passing electric
current through gases at low pressure and shiny rays were produced and were
named as Electron by G.J Stoney in since 1891. The following steps in his experiment is as follow
The following steps for electron discovery
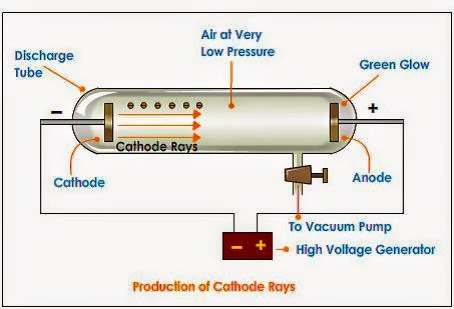
1- Construction of discharge Tube
- It Consist of a glass tube fill with a gas.
- Two metallic electrode (anode and electrode) are the opposite end of tube connected to high voltage battery.
- The pressure inside the tube is kept 10-4 atm.
- The vacuum pump is also connected to this discharge tube to reduce the pressure.
Related post
2 - Working of discharge Tube
When very high voltage current was
passed through gases shiny rays were produced from the cathode toward anode. As
these rays were generated from cathode therefore these rays are known as
cathode rays.
3 - Properties of Cathode rays (electron)
- Cathode rays traveled in a straight line and produced shadow of the object placed in their path.
- When light paddle wheel is placed in their path it is moved showing that these are actual particle in nature.
- The rays are deflected towards positive plate in electric and magnetic field proved that these rays are negatively charged.
- The speed of these rays are same as the speed of light i-e 299,792,458ms-1.
- These rays moves both as particle and waves.