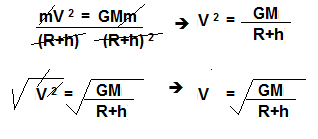Dobereiner’s triads
In
since 1820 a German Chemist John
Wolfgang Dobereiner arranged similar elements in a group of three.These elements of three groups are
known as a Dobereiner triads. Dobereiner said that when three elements in a
triads are arranged in increasing order of atomic masses then the relative
atomic mass of the middle one is very relative or close to the average of other
two elements.
Triad
|
Triad
|
||||
1
|
Cl35.5
|
1
|
Li6.9
|
||
2
|
Br79.9
|
81.2 (Br)
|
2
|
Na23
|
23 (Na)
|
3
|
I 126.9
|
3
|
K39.1
|
||
Newland’s Octaves
In since 1865 an English Chemist John
Newland proposed his law of octaves. He said that if the elements are arranged
increasing order of atomic masses then the every eight elements starting from
any point will approximately same properties of the first.
Newland’s Octaves
|
|||||||
Element
|
Li
|
Be
|
B
|
C
|
N
|
O
|
F
|
Atomic masses
|
7
|
9
|
11
|
12
|
14
|
16
|
19
|
Element
|
Na
|
Mg
|
Al
|
Si
|
P
|
S
|
Cl
|
Atomic masses
|
23
|
24
|
27
|
28
|
31
|
32
|
35
|
Element
|
K
|
Ca
|
|||||
Atomic masses
|
39
|
40
|
|||||
You also May Know